VISERA ELITE III VIDEO SYSTEM CENTER OLYMPUS OTV-S700
OTV-S700 UPGRADE PACK IR MAJ-2512
VISERA ELITE III LED LIGHT SOURCE OLYMPUS CLL-S700
4K CAMERA HEAD OLYMPUS CH-S700-XZ-EA
EVIS X1 VIDEO SYSTEM CENTER OLYMPUS CV-1500
POWERSEAL CURVED JAW SEALER & DIVIDER, DOUBLE-ACTION
IR TELESCOPE 5.4MM WAIR530A
1. All-in-One System
VISERA ELITE III system integrates the 3D and infrared imaging functions from VISERA ELITE II, and the 4K imaging function from VISERA 4K UHD system. Designed to provide optimal surgical visualisation for multiple specialties including Bariatric, Upper GI, Colorectal and Gynecological surgery. In addition to 3D and 4K imaging, VISERA ELITE III offers impressive fluorescenceguided surgery, well-proven Narrow Band Imaging (NBI), Yellow Enhancement (YE) mode all in one system.
True 4K image quality with EDOF and CAF
The VISERA ELITE III camera head can provide true 4K images through Sony’s exclusive 4K image sensor. It provides a better surgical image to surgeons and helps them distinguish finer tissues more easily.
VISERA ELITE III camera head is also equipped with EDOF (Extended Depth of Field) for greater depth of field and C-AF (Continuous Auto-Focus) for automatic focus acquisition.
This offers an advantage in maintaining clear views. Furthermore, allowing for a better flow of procedures with little interruption.
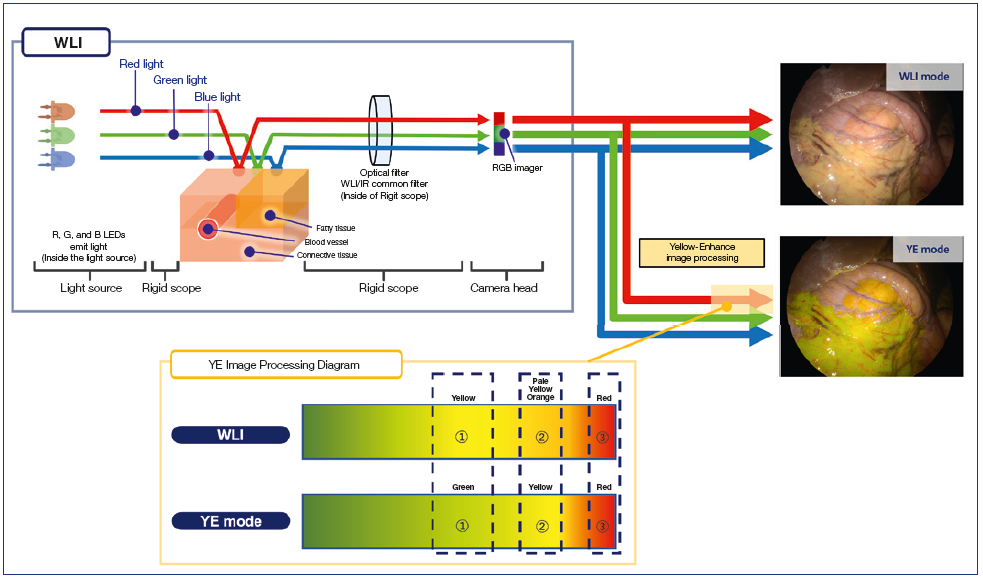
Yellow Enhancement
Yellow Enhancement (YE) is a newly added default function with VISERA ELITE III. It emphasizes yellow and is designed to support identification of structures. In YE mode, the system performs a color conversion that make orange-yellow tissue appear clearly yellow. This enhances the contrast to anatomical structures of interest.
2. The Fundamentals of ICG Fluorescence Imaging
Characteristics of ICG
When protein-bound indocyanine green (ICG) is irradiated with excitation light around 760 nm, it emits fluorescence with a peak around 830 nm. Since this band is not easily absorbed by hemoglobin or water, objects deep in connective tissue 5 to 10 mm thick can be visualized by imaging with a near-infrared observation device equipped with appropriate filters. Intraoperative fluorescence imaging is particularly effective with hepatobiliary and pancreatic surgery as these can take advantage of the characteristics of ICG, which is taken up by hepatocytes and excreted in bile after intravenous injection.
History of ICG Fluorescence Imaging Development
Fluorescence imaging using ICG was originally applied clinically to fundus angiography in ophthalmology. At the turn of the century, it was quickly introduced to the operating room for applications such as blood flow evaluation in coronary artery bypass surgery and sentinel lymph node biopsy for breast cancer. In the field of hepatobiliary and pancreatic surgery, ICG fluorescence imaging has been applied in a wide range of applications, including biliary angiography and liver tumor delineation, starting with the liver zone identification method reported in 2008. Undoubtedly, the background for the development of these techniques is the widespread use of camera systems that enable IR observation during laparoscopic surgery and the improvement of image quality.
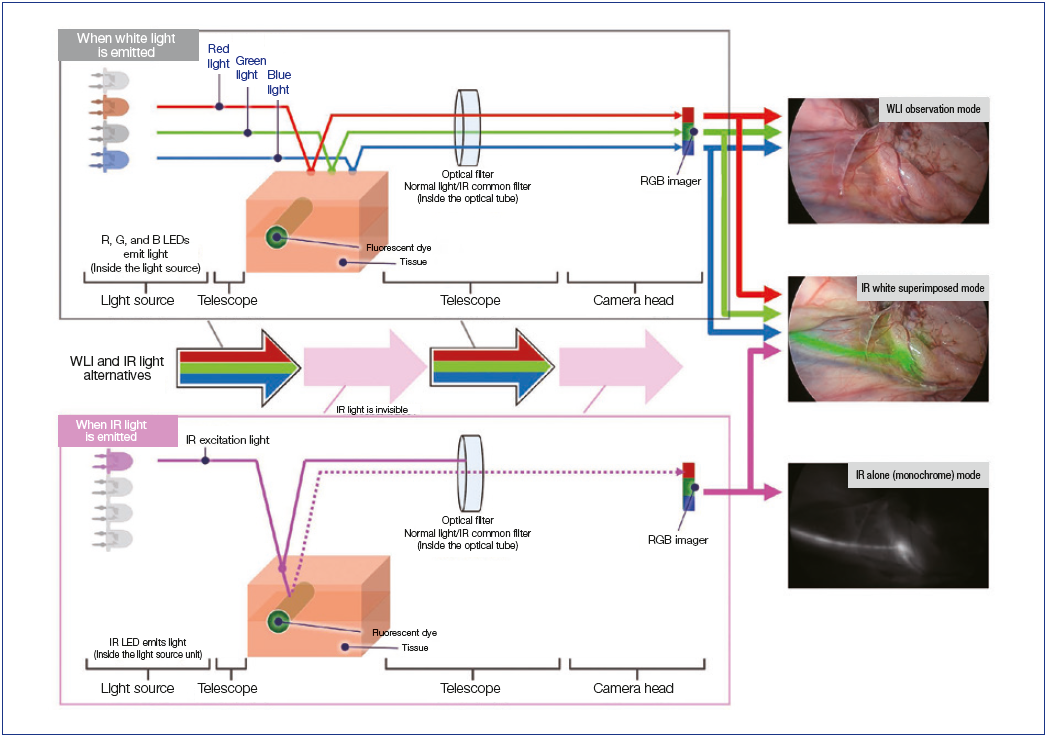
IR+4K+3D Surgical Endoscopy System VISERA ELITE III Features
Introduced in 2022, the long-awaited VISERA ELITE III imaging system provides IR observation with 4K image quality and also supports 3D. For fluorescence imaging, the VISERA ELITE III is equipped with not only the monochrome (MODE 1) and superimposed 2-tone color (MODE 2) image modes offered by the VISERA ELITE II, but also a third mode that superimposes fluorescence images on 4K full color images (MODE 3), which can be easily switched by operating the camera head as required for the application. Although not directly related to IR observation, the camera head is lightweight despite its many functions, while its extended depth of field and continuous autofocus eliminate the need for frequent focus adjustments, which is a drawback of 4K.
3. Bariatric Surgery
Introduction
The efficacy of bariatric surgery in combating metabolic syndrome and obesity remains unparalleled, with sleeve gastrectomy emerging as the most performed surgical approach, surpassing the traditional Laparoscopic Roux-en-Y gastric bypass(LRYGB). The surging popularity of alternate bypass methodologies, such as one anastomosis gastric bypass (OAGB) and single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S), further contributes to this evolving landscape. Notably, the cumulative number of bariatric interventions conducted worldwide has demonstrated a marked escalation, underscoring these procedures’ widespread recognition and adoption as a cornerstone in managing metabolic syndrome and obesity.
Technical success and lowering morbidity in sleeve gastrectomy
and standard bypass procedures
- Addressing leaks and bleeds arising from staple lines and anastomoses is a paramount concern in bariatric surgery due to their substantial morbidity. Surgeons employ a range of adjunctive measures during procedures to mitigate these complications. Nonetheless, the meticulous practice of dissection and the attainment of adequate haemostasis remain pivotal for success.
- For optimal outcomes in weight loss and the mitigation of reflux, the symmetrical construction of sleeve gastrectomy and the vertical pouch within procedures such as One Anastomosis Gastric Bypass (OAGB) and Laparoscopic Rouxen- Y Gastric Bypass (LRYGB) holds significant importance. Notably, these approaches prove advantageous by offering enhanced results and minimising the incidence of reflux. Rectifying any asymmetrical dilation becomes imperative in cases necessitating revisions from prior gastric bands or anti-reflux procedures.
- The complexities associated with revision surgery after Adjustable Gastric Banding present further challenges, primarily due to fibrous scar tissue and stomach folding. Notably, during revisions after an adjustable gastric band procedure, the inadvertent compromise of the vascular supply to the upper stomach can lead to developing ischemic areas, consequently elevating the risk of leaks.
- Recognition and proactive management of hiatus hernias during both primary and revision procedures are highly recommended strategies. By incorporating these measures, the potential for reflux-related complications can be notably diminished, thereby enhancing patient outcomes and minimising associated risks.
Aims of any enhancement technology
- Guide safe surgery to prevent adverse events.
- Intraoperative recognition of complications allows for the opportunity to rectify.
- Add confidence in the quality of surgery to accelerate recovery and progress post-operatively.
ICG in bariatric surgery
- Assessment of tissue perfusion
- Assessment of perfusion post-resection at risky areas.
- Assessment of anastomosis perfusion in gastrojejunostomy , entero-enterostomy and duodenojejunostomy
4. Sleeve gastrectomy
Mobilisation of the stomach
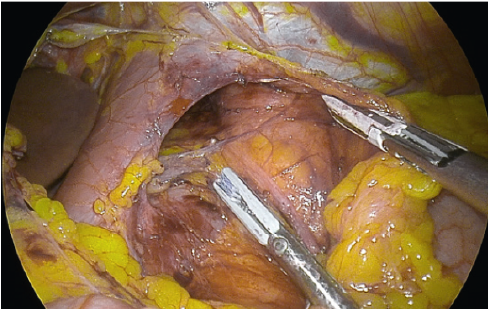
- The stomach is freed from the greater curve starting approximately 3- 5 cm from the pylorus. Effective sealing of the vessels from the gastroepiploic arcade to the greater curve can be achieved by a bipolar energy device. The short gastric and posterior gastric vessels are sealed.
- Caution should be taken when using energy devices, particularly at the antrum and the angle of His region. Thermal spread can
damage tissues and cause late ischemia and leak. The areas at the highest risk are at the beginning and end of the staple line.
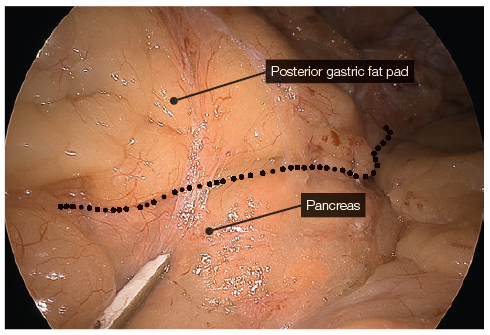
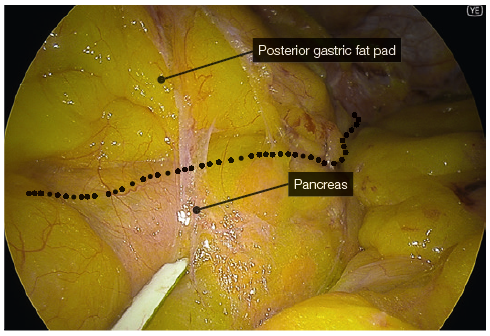
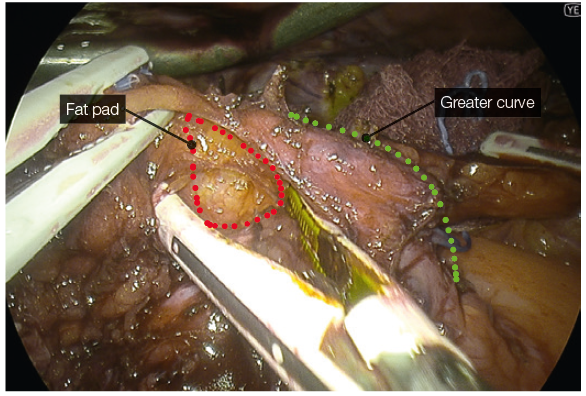
Bipolar sealing and cutting devices with these characteristics offer safer and more speedy dissection. - The posterior gastric fat pad is separated from the LN pack and fat over the splenic artery and the left pillar of the hiatus. This allows for complete mobilisation of the posterior stomach, eliminating a posterior gastric pouch, therefore ensuring symmetry during resection.
Tips and caution
- Using C-AF facilitate continuous zooming in and out for the operator. This facilitates efficient and careful dissection of the short gastric, especially at the superior pole of the spleen, where they can be very short.
- Deeper and wider bites can result in injury to a polar vessel or bleeding due to injury of larger vessels.
- Using a 5mm 30-degree telescope allows the surgeon to move between ports to get the best views of high-risk areas. Operative views in this case report have been obtained using a 5mm IR-compatible telescope.
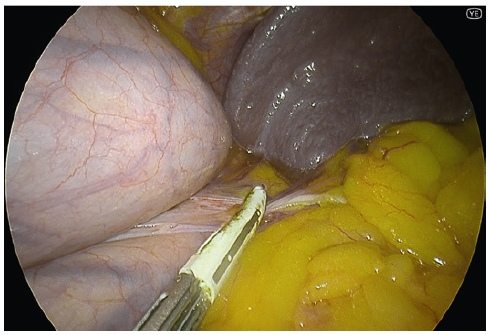
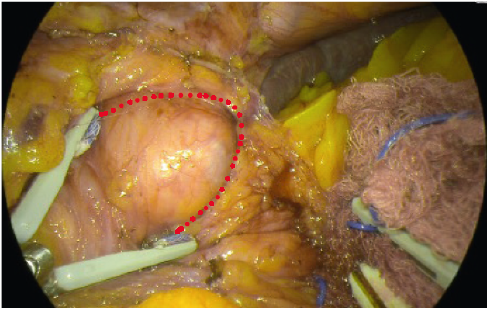
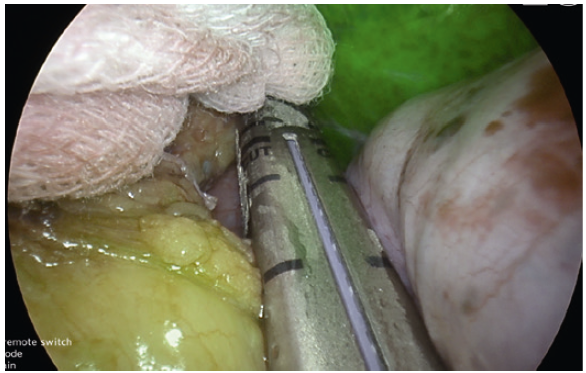
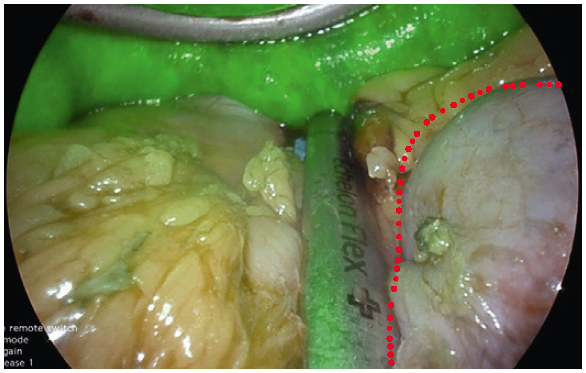
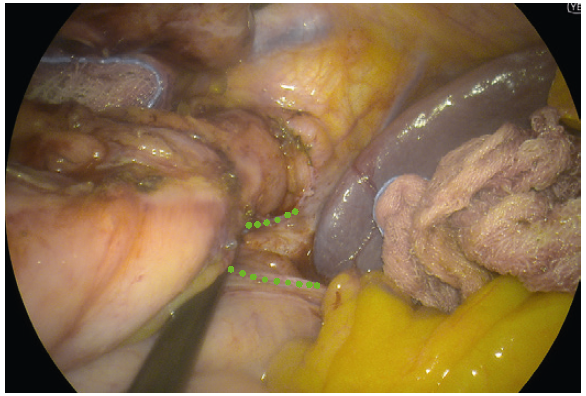
- YE helps further delineate the posterior fat pad from the hiatal pillar and pancreas (Fig. 2-3).
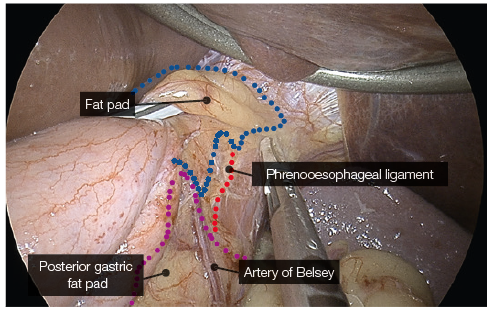
Identification of phreno-oesophageal ligament and
assessing the status of a hiatus hernia.
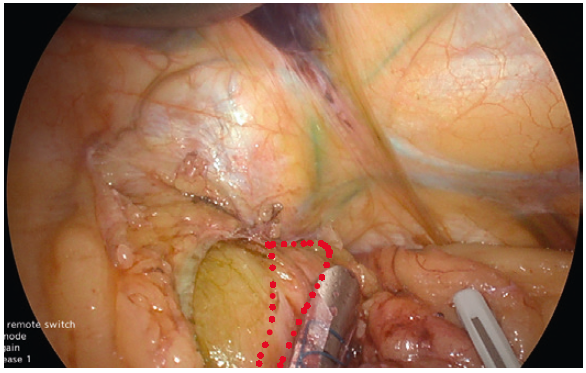
- Clear visualisation of the phreno-oesophageal ligament and confirmation of intraabdominal oesophagus reduces the risk of reflux.
- Care must be taken not to disrupt the ligament to avoid the creation of weakness and increase the risk of herniation.
Tips and caution
- Avoid energy devices with high thermal spread. Contact with heat in this area can damage the tissues and increase the risk of
vascular complications and leaks at the top of the staple line, which is the highest site of leaks post-sleeve gastrectomy. - When exposing the angle of His anteriorly, it is helpful to have a device that offers bipolar cautery on the surface to limit the capillary bleed that can be encountered when mobilising the fat pad without generating heat that penetrates the stomach wall and
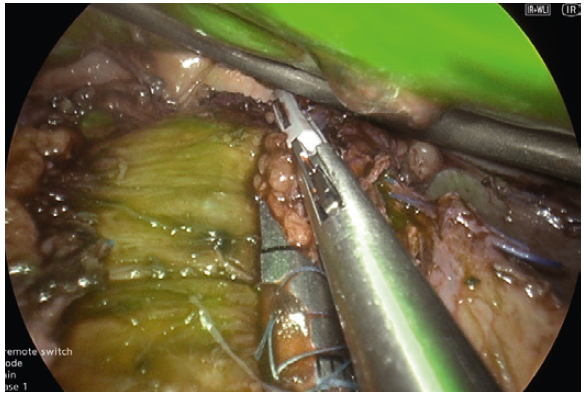
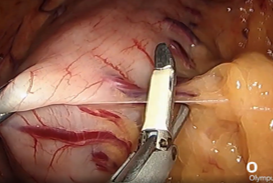
prevents potential thermal injury. - We use the Powerseal device, which has these advantages of limited thermal spread, a curved fine tip that safely helps dissect tissues and excellent and rapid sealing of vessels.
- Using YE increases the ease of identification of the phreno-oesophageal ligament (Fig. 5).
Assessment of perfusion
- The use of ICG in bariatric surgery is well-established, particularly in cases where the risk of disruption of the collateral circulation of the gastric conduit or the small bowel is high.
- Revision surgery also poses an increased risk due to scaring or complications associated with previous surgery in the area. The extent of vascular compromise of the target area of surgery is often not known until intraoperative dissection is carried out.
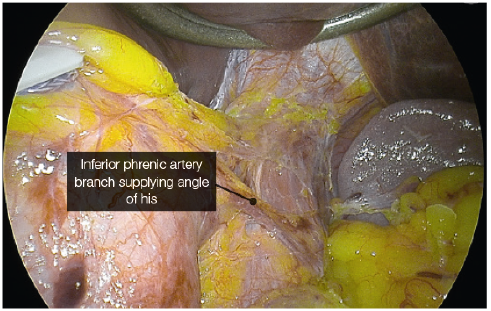
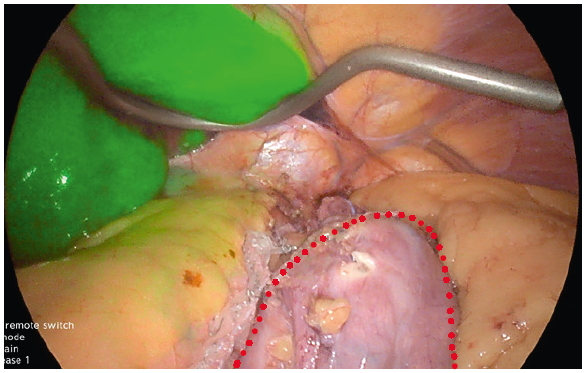
- In sleeve gastrectomy, the upper end of the staple line is close to the angle of His. The blood supply to this area is variable and can predominately come from a branch of the inferior phrenic artery. This is the most common site of leaks. Many factors have been attributed to causing a leak at this site, with ischemia being the likely precipitating factor.
- Increasing the distances from this area during resection can decrease the leak rate (wider bougie ) but may reduce the sleeve’s efficacy and, therefore, weight loss. A balance must be struck.
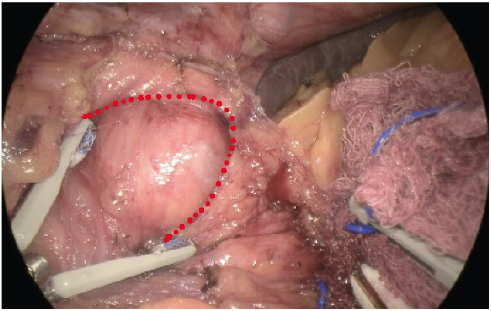
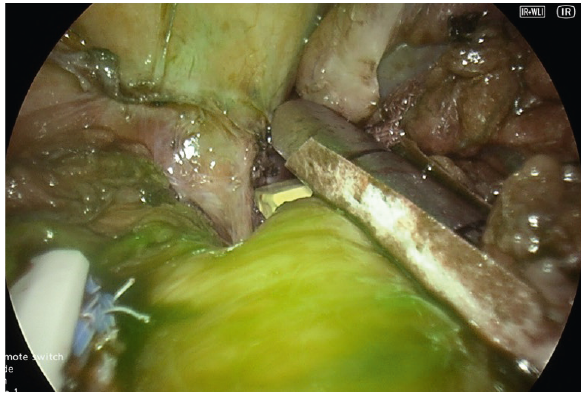
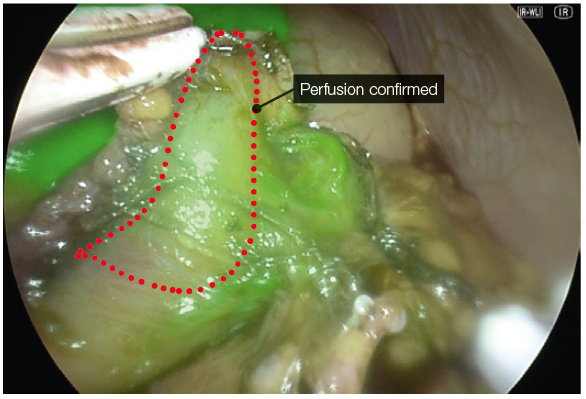
- We use ICG to confirm adequate perfusion of the stomach tip after clamping the stapler device at the last firing whilst being close to a 36fr bougie at the angle of His. This often leaves a 0.5mm distance from the angel without vascular compromise and excess gastric fundus (dog ear)
- IG has been used to confirm perfusion for fundoplication during the Nissen sleeve and partitioned fundus during the Mengen Strasse Mill procedure (not demonstrated in this case report.
Tips and caution
- ICG penetrates the tissues and does not leave the tissues for a considerable amount of time. When tissue perfusion is assessed, we need to recognise that high doses of ICG can prevent repeated assessment due to tissue saturation.
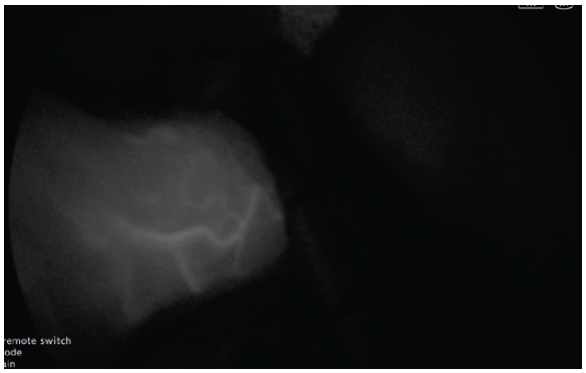
- Very Low dose of ICG, e.g. 1 mg dose, does not saturate the tissue enough to give a reliable assessment (Fig. 10).
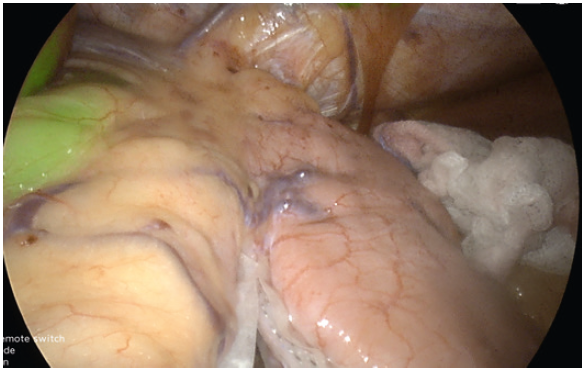
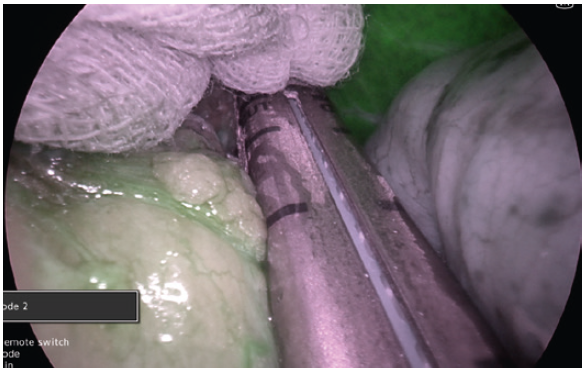
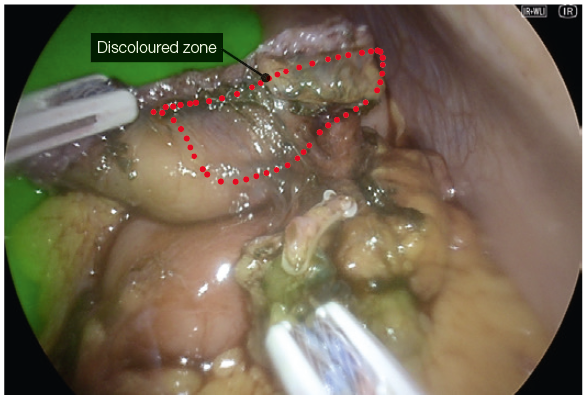
- In perfusion assessment of the gastric sleeve, small bowel or duodenum, we use 2 mg bolus injections. The perfusion can be assessed live under IR modes. During hybrid (superimposed IR and 4K colour) mode, the operator can visualise the tissue enhancement and the vessels on the lesser curve or mesentery (Fig. 11).
- After 5 minutes, the vascular enhancement diminishes, but the tissue retention of the ICG remains (Fig. 15).
- In repeated assessments, a further 2mg dose can be administered, and the reperfusion can be assessed. This differential incremental reperfusion can be seen easily in the IR mode, less so in the two-colour superimposed mode (Fig. 12). A useful adjustment is lowering the gain to low. This further allows easier imaging of the change in reperfusion if further doses are administered (Fig. 13).
- Caution must be noted when the reflection of the liver ICG retention on the gastric tissue can be misinterpreted as enhancement of the gastric wall. Covering the liver’s undersurface with gauze can confirm this.
5. Revision from Band to Sleeve gastrectomy
Essential steps in revision from band to sleeve or
vertical pouch in bypass surgery
- Form a healthy symmetric sleeve or pouch and apply the same principles as a primary surgery.
- Unfolding the stomach completely anteriorly requires freeing of the scar and preserving the lesser curve arcade.
- Freeing the posterior aspect of the stomach particularly the band tunnel posteriorly to eliminate tension and potential pouch
- Clear identification fo angle of His and phreno-oesophageal ligament.
Tips and caution
- YE aids the surgeon in differentiating the planes anteriorly and posteriorly
- C-AF and 4K offers better enhancement and contrast with ability to zoom in and out seemingly
- ICG further assures adequate perfusion of the upper end of the conduit. It can also be used to identify pedicles of major gastric vessels when the inflammatory process causes fusion of planes posteriorly.
6. Anastomosis assessment
- In Bypass surgeries the divided gastric conduit tip is at risk of ischemia.
- Small bowel perfusion is also at risk during mesenteric mobilisation.
- Check perfusion of staple ends before anastomosis is reconstructed.
- When a stapled gastrojejunostomy is performed, the zone between the vertical gastric staple line and the anastomotic staple line is at risk of ischemia.
- Check perfusion of the anastomosis with ICG post-reconstruction
Tips and caution
- It is preferred to assess perfusion before reconstruction to avoid redoing an anastomosis
- In stapled anastomosis, not all devices have enough compression when closed to restrict perfusion enough to aid in the identification of ischemic zones.
- We prefer to use a bowel clamp to mimic the stapling device before injecting ICG and assessing perfusion. If ischemia is noted, the clamp is moved towards the lesser curve and re-tested.
- The doses and IR modes used are similar to sleeve perfusion repeated dose assessments. 2 mg bolus injections of ICG are used.
7. Endoscopy during Bariatric surgery
- Gastroscopy is essential in preoperative assessments in patients considered for bariatric surgery.
- IFSO guidelines recommend gastroscopy for patients planning to undergo a bariatric procedure.
- Intraoperative gastroscopy helps to identify the location of the Gastroesophageal junction and assess the presence and repair of hiatal hernias.
- It also helps confirm the symmetry of the sleeve and the absence of twisting during insufflation.
- Identification of severe angulation or twisting can be rectified during the index operation.
- It offers the opportunity to assess for bleeding from the mucosa side of the staple line or anastomosis.
Tips and caution
- Using CO2 for insufflation is recommended to avoid patients’ discomfort from prolonged distention of the bowel with air.
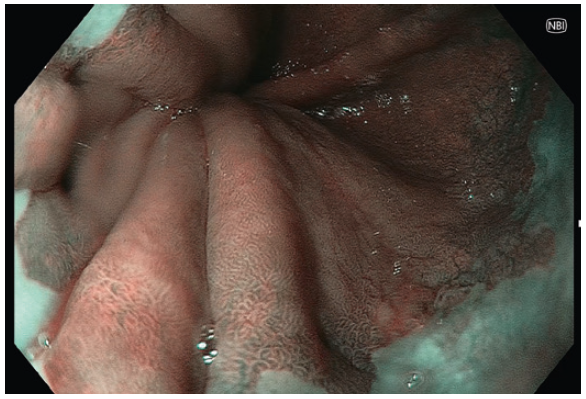
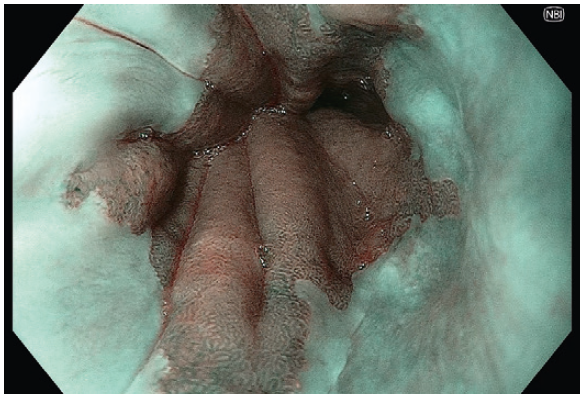
- Using NBI helps clear the demarcation of the Squamocolumnar junction.
- Near Focus and NBI further delineate the vertical submucosal vessels and gastric cardia mucosal pit pattern to confirm the gastroesophageal junction.
- In conjunction with laparoscopy, an inside-to-outside view confirms anatomy.
- RDI allows for accurate assessment of mucosal bleeding, which can be present in 2.5% of cases.
Conclusion
Bariatric surgery remains the most effective modality for the treatment of metabolic syndrome and obesity. Sleeve gastrectomy is now the preferred method over traditional procedures like the Laparoscopic Roux-en-Y gastric bypass (LRYGB), thanks to its efficacy and lower rates of complications such as leaks and bleeds. The meticulous surgical technique, including careful dissection and maintaining haemostasis, is crucial for success. The symmetrical construction of the gastric sleeve minimises reflux, reduces side effects and ensures effective weight loss, particularly after revision surgery following the removal of the adjustable gastric band.
Enhancement technologies in bariatric surgery aim to guide safer surgeries, allow for intraoperative identification and rectification of complications, and boost confidence in the surgery’s quality to aid post-operative recovery. Indocyanine Green (ICG) fluorescence is a valuable tool for assessing tissue perfusion and ensuring the viability of the gastric sleeve and anastomoses, which is crucial for reducing the risk of ischemic complications.
Specific technical considerations in sleeve gastrectomy during the mobilisation of the stomach, with careful handling of high-risk areas, using energy devices with minimal thermal spread, and intraoperative endoscopy are recommended to help prevent, identify and rectify potential issues such as ischemia, twisting or bleeding.
References
Brown, W.A., Johari Halim Shah, Y., Balalis, G. et al. IFSO Position Statement on the Role of Esophago-Gastro-Duodenal Endoscopy Prior to and after Bariatric and Metabolic Surgery Procedures. OBES SURG 30, 3135–3153 (2020). https://doi.org/10.1007/s11695-020-04720-z
Gagner M, Hutchinson C, Rosenthal R. Fifth international consensus conference: current status of sleeve gastrectomy. Surg Obes Relat Dis. (2016) 12(4):750–6. doi: 10.1016/j. soard.2016.01.022
Alhaj Saleh A, Janik MR, Mustafa RR, Alshehri M, Khan AH, Kalantar Motamedi SM, Rahim S, Patel I, Aryaie A, Abbas M, Rogula T, Khaitan L. Does Sleeve Shape Make a Difference in Outcomes? Obes Surg. 2018 Jun;28(6):1731-1737. doi: 10.1007/s11695-017-3087-6. PMID: 29313277.
Balla A, Corallino D, Quaresima S, Palmieri L, Meoli F, Cordova Herencia I, Paganini AM. Indocyanine Green Fluorescence Angiography During Laparoscopic Bariatric Surgery: A Pilot Study. Front Surg. 2022 May 26;9:906133. doi: 10.3389/fsurg.2022.906133. PMID: 35693301; PMCID: PMC9178117.
Frattini F, Lavazza M, Mangano A, Amico F, Rausei S, Rovera F, Boni L, Dionigi G. Indocyanine green-enhanced fluorescence in laparoscopic sleeve gastrectomy. Obes Surg. 2015 May;25(5):949-50. doi: 10.1007/s11695-015-1640-8. PMID: 25736231.
Hsu A, Mu SZ, James A, Ibrahim MA, Saber AA. Indocyanine Green in Bariatric Surgery: a Systematic Review. Obes Surg. 2023 Nov;33(11):3539-3544. doi: 10.1007/s11695-023-06801-1. Epub 2023 Sep 15. PMID: 37713041.
Wityk M, Dowgiałło-Gornowicz N, Feszak I, Bobowicz M. Fluorescence use in minimally invasive metabolic and bariatric surgery – a systematic review of the literature. Langenbecks Arch Surg. 2023 May 30;408(1):216. doi: 10.1007/s00423-023-02955-9. PMID: 37249703; PMCID: PMC10229673.
van Manen L, Handgraaf HJM, Diana M, Dijkstra J, Ishizawa T, Vahrmeijer AL, Mieog JSD. A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J Surg Oncol. 2018 Aug;118(2):283-300. doi: 10.1002/jso.25105. Epub 2018 Jun 24. PMID: 29938401; PMCID: PMC6175214.
Olmi S, David G, Cesana G, Ciccarese F, Giorgi R, De Carli S, Uccelli M. Modified Sleeve Gastrectomy Combined with Laparoscopic Rossetti Fundoplication and Vascularization Assessment with Indocyanine Green. Obes Surg. 2019 Sep;29(9):3086-3088. doi: 10.1007/ s11695-019-03970-w. PMID: 31115851.
Biancucci A, Fassari A, Lucchese S, Santoro E, Lirici MM. Use of quantitative indocyanine green near-infrared fluorescence imaging in bariatric surgery: early results. Minim Invasive Ther Allied Technol. 2023 Oct;32(5):249-255. doi: 10.1080/13645706.2023.2197049. Epub 2023 Apr 11. PMID: 37039717.
*Disclaimer* Any content or information (“Content”) presented herein is illustrative in nature and does not guarantee or represent specific information, outcomes, or results. Olympus Medical Systems Corp. and its parents, subsidiaries, affiliates, directors, officers, employees, agents, and representatives (collectively “Olympus”) does not represent to or warrant the accuracy or applicability of the Content. Under no circumstances shall Olympus be liable for any costs, expenses, losses, claims, liabilities, or other damages (whether direct, indirect, special, incidental, consequential, or otherwise) that may arise from, or be incurred in connection with, the Content or any use thereof.