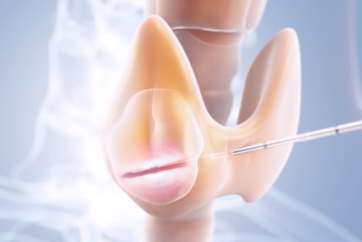
TREATMENT OF HABITUAL SNORING – STIFFENING OF SOFT PALATE
Habitual snoring is frequently caused by vibrations in the soft palate, the uvula, or the tissue in the area of the palatine arches. It is recommended to properly diagnose a sleep-related breathing disorder before you start the procedure: If the patient has other conditions such as obstructive sleep apnea, additional treatments might be necessary. In the following chapter, it is demonstrated how to use bipolar radiofrequency-induced thermotherapy (RFITT) to treat habitual snoring caused by the soft palate.
01 | Anesthesia and Medications
The soft palate can be treated with RFITT using local anesthesia.
Apply a topical anesthetic like lidocaine gel or spray (ten percent) to the palate and uvula of your patient to counteract their gag reflex and reduce the pain of the injections.
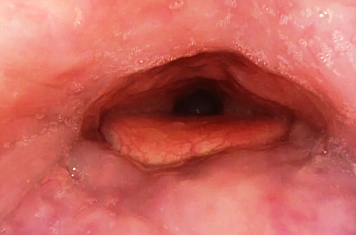
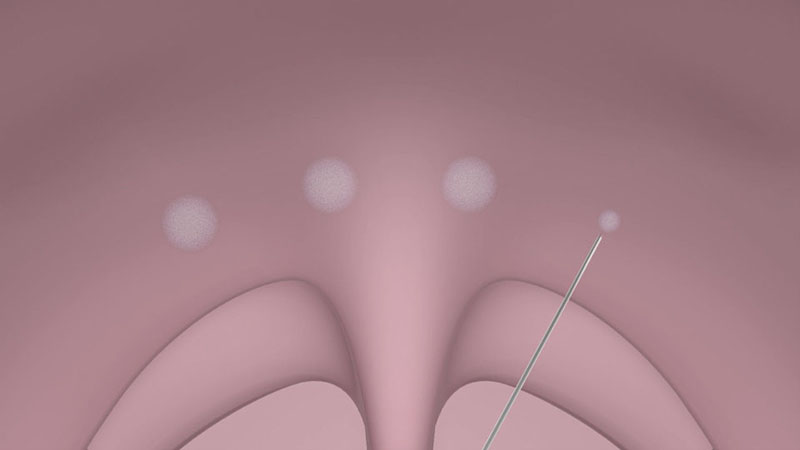
For local anesthesia, the injection of 0.5 ml of a two percent lidocaine solution with adrenaline is recommended at two points paramedially below the border between the hard and soft palates [Figure 01a/b].
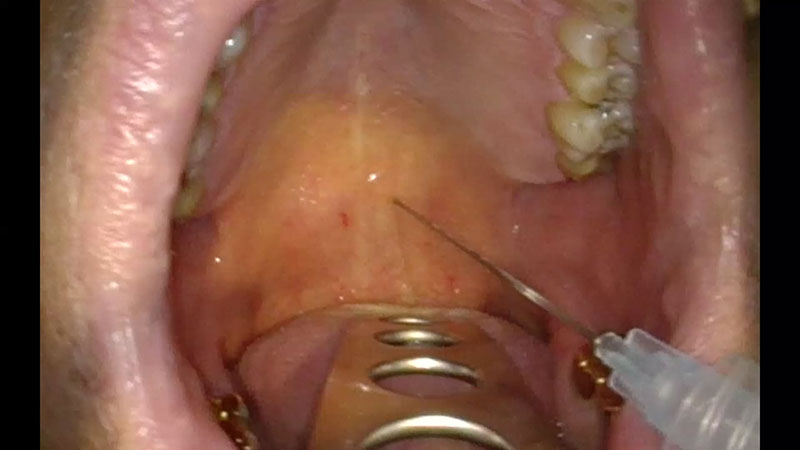
These injections also enlarge the volume of the palatine tissue during the procedure and reduce the risk of damage to the mucous membrane.
After three to five minutes, additional four injections are necessary in the lower part of the soft palate (0.5 ml on each side) [Figure 02a/b].
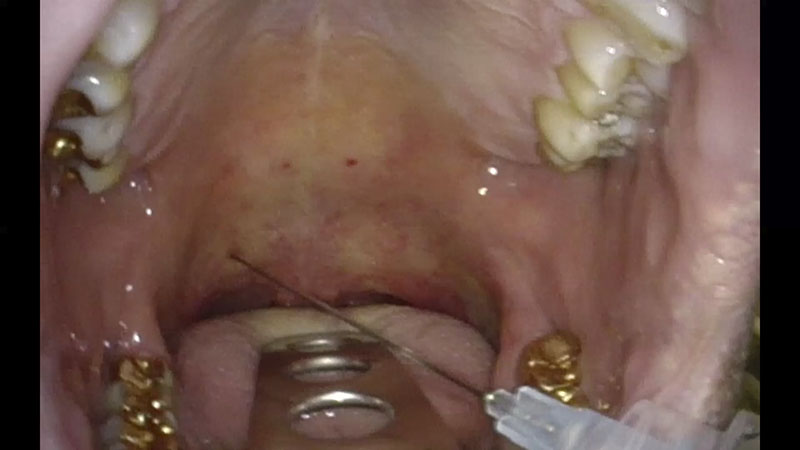
02 | Treatment of the Soft Palate (with RFITT)
Power settings: A power setting of 12 watts (mode: PureRFITT) is recommended for treating the soft palate. The power
setting on your control unit determines the extent of coagulation. The lower the power setting, the longer the application
time and the greater the extent of coagulation. The higher the power setting, the smaller the area of the coagulation.
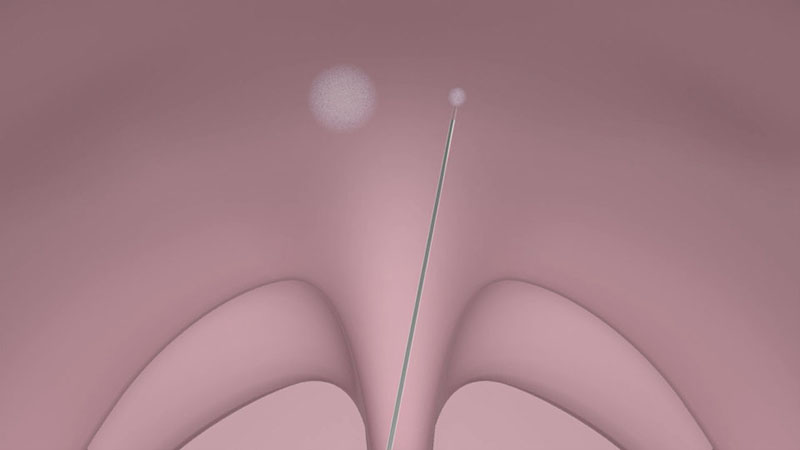
Use the CelonProSleep plus applicator to treat the soft palate.
Insert the tip of the applicator into the palatal muscle until the applicator’s insulation tube touches the tissue [Figure 03a/b].

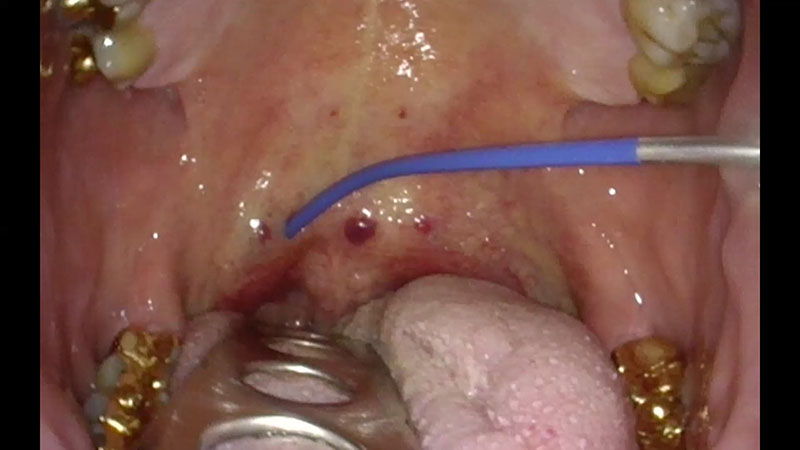
After positioning the applicator, activate the power supply by pressing the foot switch. You can monitor the status of coagulation via the acoustic signal.
Make sure that the tip of the applicator is located as precisely as possible in the middle of the tissue (soft palate muscle). To avoid damage to the mucous membrane, do not place the coagulations too laterally or inferiorly. Furthermore, to prevent unnecessary swelling, do not insert the needle tip up into the uvula.
The power supply is reduced automatically to prevent overdose effects. If your patient has a wide and very thick soft palate, you can increase the number of coagulations. One insertion with three coagulations on each side at regular intervals is recommended [Figure 04a/b].
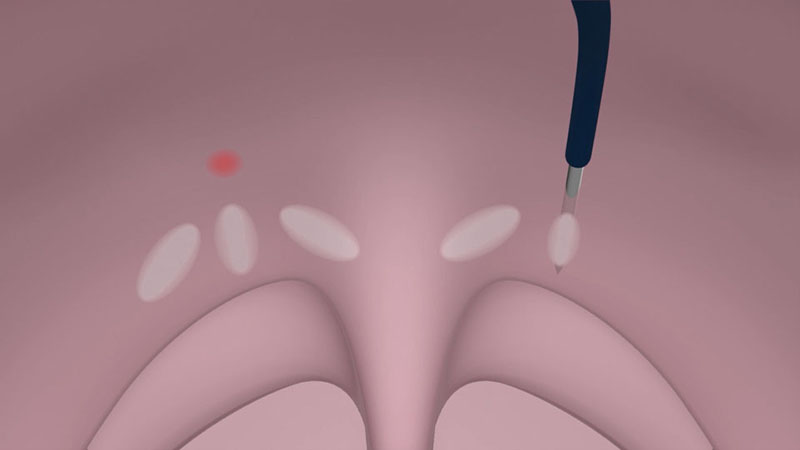

Note:
- Should the mucous membrane becomes pale or white during the application, the power output must be stopped immediately. The applicator must be repositioned, as necrosis of the mucous membrane can occur otherwise, and, in the worst case, ulceration.
- In case of a very thin soft palate, a slightly higher power output setting may be helpful to create smaller lesions.
03 | Therapeutic Effect
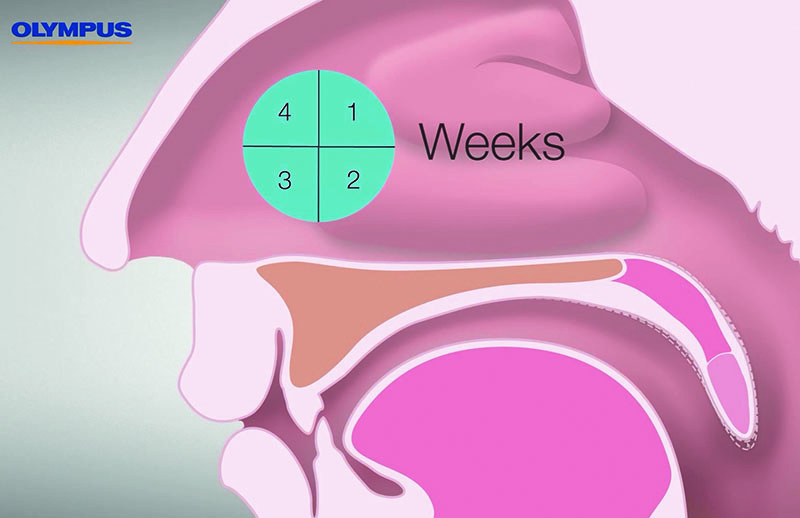
The coagulation results in a local denaturation of the treated tissue area. Within approximately four weeks, you can expect a visible reduction in volume accompanied by a tightening of the palatine tissue as a result of the body’s resorption process, and the formation of scar tissue [Figure 05].
04 | Postoperative Care
To ensure that swelling is reduced more swiftly and to avoid pain, ibuprofen or diclofenac are recommended. A follow-up visit can be scheduled two to three days after the procedure to rule out any infections.
Note:
- Clinical experience indicates that an additional coagulation treatment of the soft palate is usually necessary to achieve optimal results. When conducting additional treatments, the CELON applicator should be placed between the scarred areas of the tissue.
- It is recommended to wait at least four weeks before conducting a follow-up intervention.
- Content Type