Author

Mingyon Mun, M.D., Ph.D., Department of Thoracic Surgical Oncology,
Cancer Institute Hospital of Japanese Foundation for Cancer Research
Biography
Graduated from Wakayama Medical University in 1996. After surgical training at Toranomon Hospital in 1997, he mastered thoracoscopic surgery working as a surgeon at the Respiratory Center of the same hospital from 2003. He became a Doctor of Medical Science at Kitasato University in 2007. Since 2008, he has been working at the Cancer Institute Hospital of JFCR, where he has introduced thoracoscopic surgery. He has occupied his present post since April 2013, working on minimally invasive surgery for lung cancer.
Introduction
Thoracoscopic lung segmentectomy is one of the minimally invasive surgical procedures for peripheral small lung tumors and preserves pulmonary function. While it allows an adequate surgical resection margin to be ensured more easily than partial lung resection, a way has to be devised for identification of intersegmental borders. A common approach for the identification of intersegmental borders is the inflation-deflation method using segmental bronchi. However, it has drawbacks including: 1 stable inflation-deflation lines cannot be created in patients with emphysema and those showing insufficient deflation of the lung, and 2 the inflated lung disturbs the visual field in thoracoscopic surgery. On the other hand, identification of intersegmental borders using ICG fluorescent imaging based on segmental pulmonary arteries as markers is a useful method that can visualize intersegmental borders in the deflated lung with ease and little variability.
Application to ICG Fluorescent Imaging in Lung Segmentectomy
Since that application of ICG in lung segmentectomy is an off-label use, this was carried out as a clinical research after being reviewed and approved by the IRB of our hospital. For lung segmentectomy, after treatment of the pulmonary arteries/bronchi in the segment to be resected, ICG (0.25 mg/kg) was intravenously administered. While the segment to be resected appears dark because of the lack of ICG due to dissection of the pulmonary artery, the preserved segments emit fluorescence because of ICG supplied from the intact pulmonary artery. The borders between them are recognized as intersegmental borders and marked using electrocautery. After marking, the lung parenchyma is dissected.
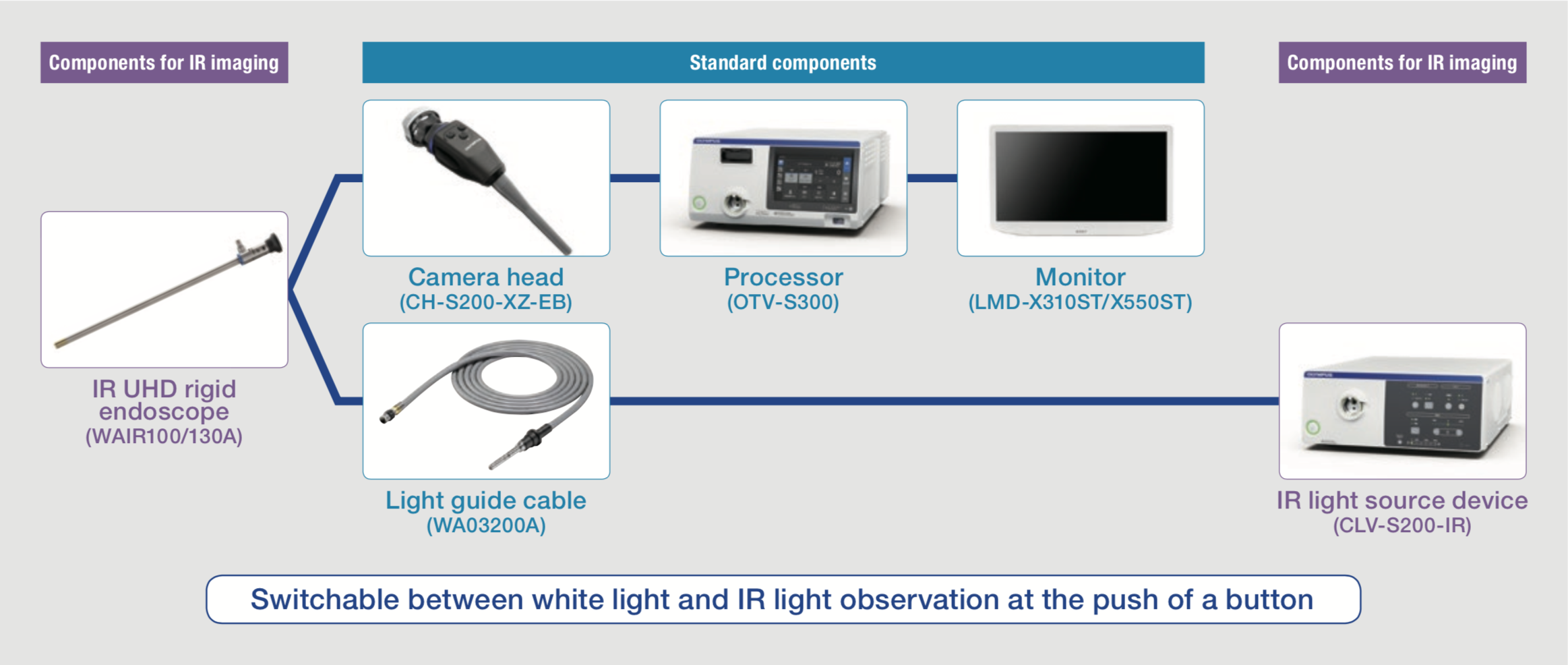
Surgical System for IR Observation
The IR observation system is shown in Figure 1. IR light source is combined with the conventional system, and it is easy to switch between white light observation and IR observation simply by pressing a button on the camera head.
Thoracoscopic Right Basal Segmentectomy Using IR Observation
1 Preoperative evaluation
In segmentectomy, it is necessary to understand subsegmental anatomy in reference to not only axial but also sagittal and coronal views of the conventional thin slice CT (TSCT), as well as to 3D reconstructed images.
2 Monitor setting and port placement
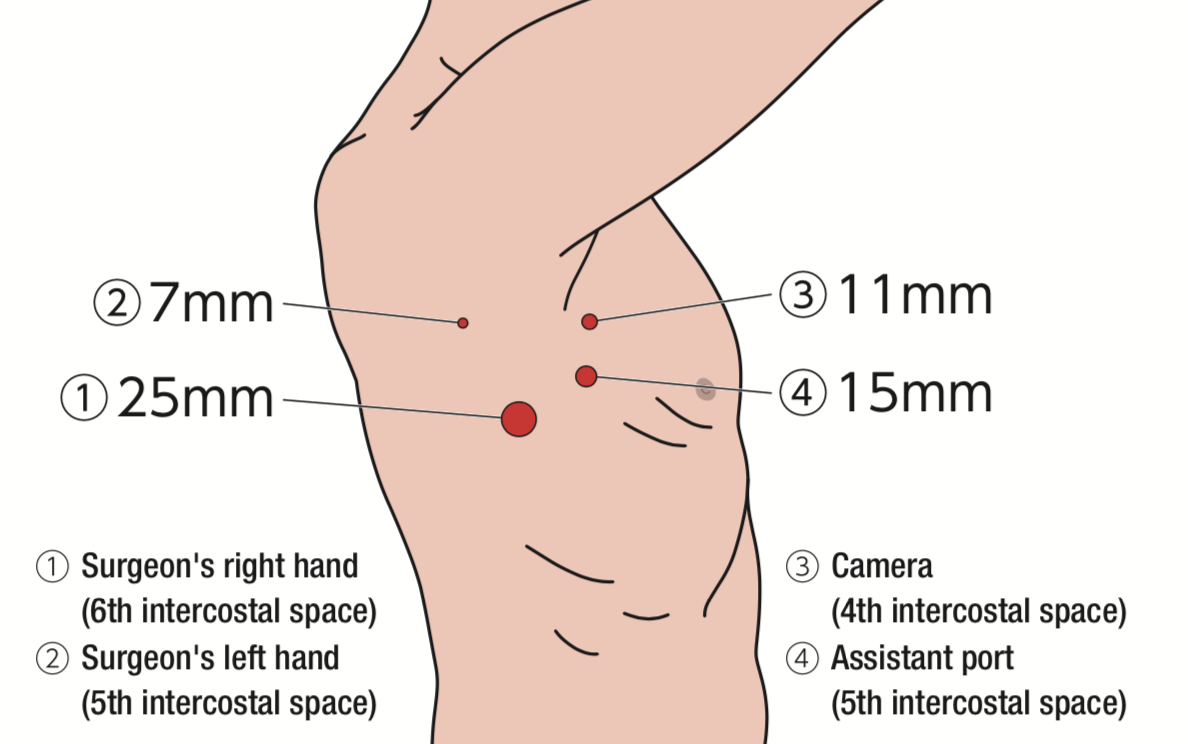
Two monitors are installed on the cranial side of the patient and one of them is placed upside-down (confronting upside-down monitor setting). By making 4 incisions (25, 15, 11, 7mm) at the same locations as in pulmonary lobectomy (Figure 2), all surgical procedures are carried out under visualization on the monitor completely.
3 Surgical procedures (right basal segmentectomy)
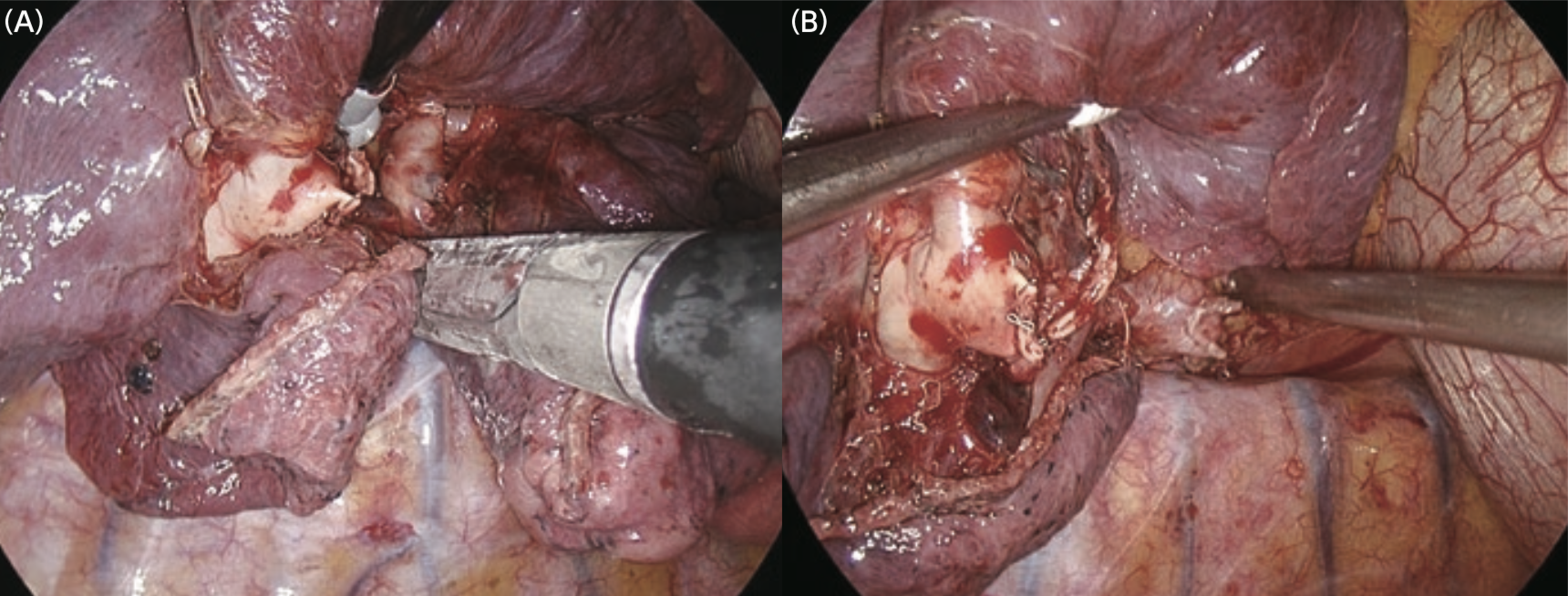
① The pulmonary ligament was dissected, and the inferior pulmonary vein was exposed.
② The interlobar pleura was opened, and the pulmonary artery was exposed.
③ Basal segment pulmonary veins and arteries were separately dissected using an endoscopic stapler.
④ The fissure between middle and lower lobe was dissected using an endoscopic stapler.
⑤ After checking the basal segment bronchi with a bronchoscope, they were dissected using an endoscopic stapler. (The blood flow from the bronchial artery in the resected segment should be blocked in advance.)
⑥ The V6b and V6c were exposed until the periphery.
⑦ ICG 0.25 mg/kg was injected intravenously (after intravenous injection of ICG, drip infusion is stopped to facilitate the rapid delivery of ICG).
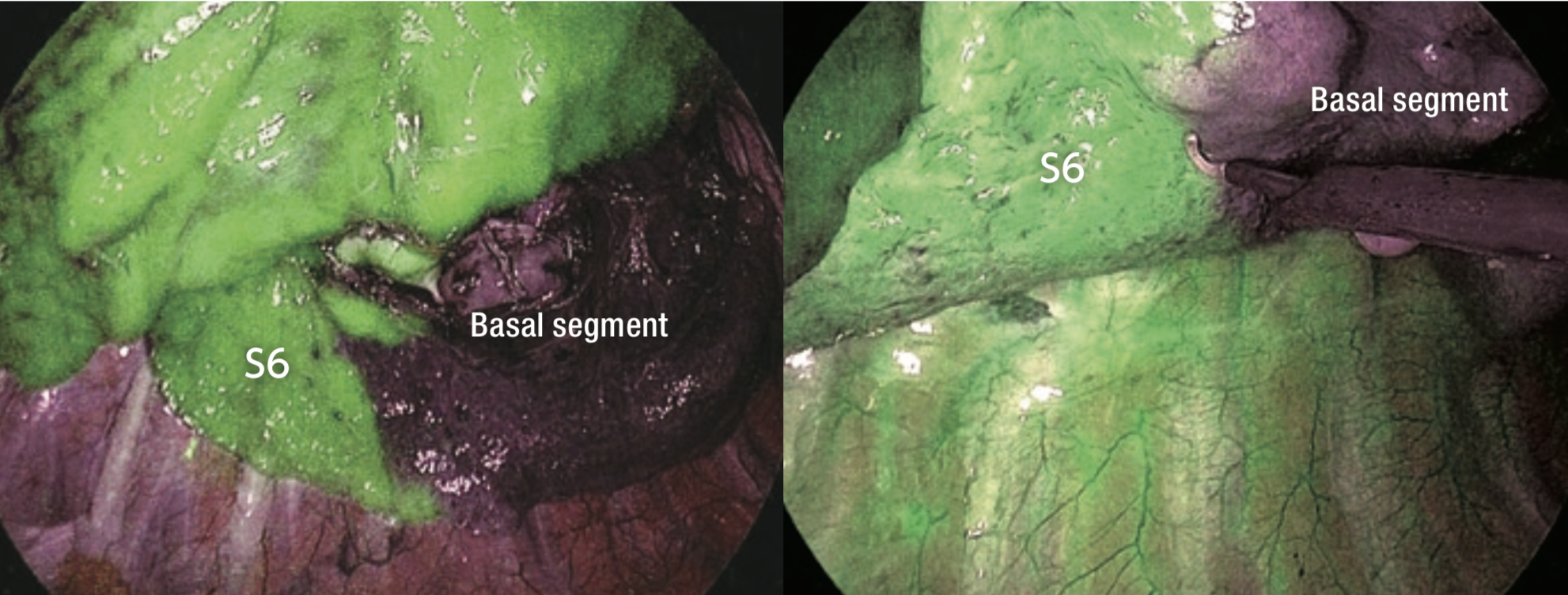
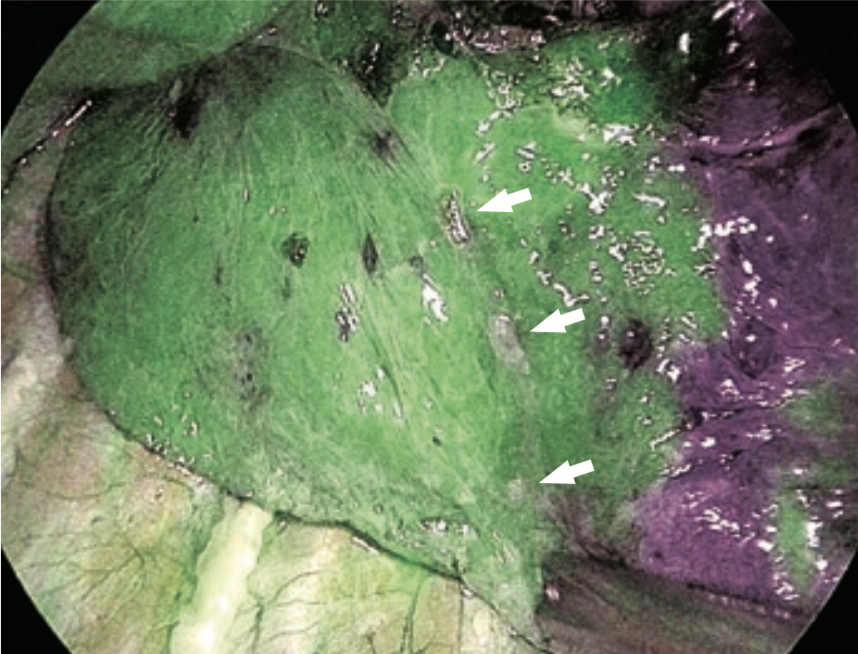
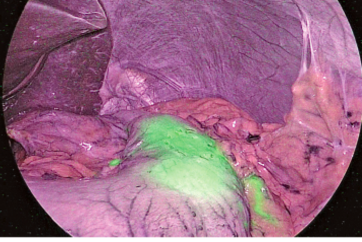
⑧ ICG fluorescence could be identified 17 seconds after intravenous injection (Figure 3). The borders on the pulmonary surface were marked using electrocautery. ICG fluorescence gradually attenuated and extended to the segment to be resected (Figure 4).
⑨ The lung parenchyma was dissected using an endoscopic stapler (Figure 5) along the marking (in the present case, the intersegmental border was dissected slightly closer to S6 in order to ensure an adequate surgical resection margin).
⑩ The specimen was retreaved. Fibrin glue and poly glycolic acid sheets were applied to the dissected area. After placement of a 20-Fr drain, the surgical wounds were closed.
Tips and Cautions in Application of IR Observation to Lung Segmentectomy
Since ICG is taken up by hepatocytes, ICG fluorescence persists for a long time in the case of liver resection. By contrast, in the lung parenchyma, ICG fluorescence gradually extends to the segments to be resected and the fluorescence intensity decreases because of transition from the pulmonary arteries to the veins and blood supply from the bronchial arteries, etc. According to the assessment of 20 cases of thoracoscopic segmentectomy performed at our hospital using the ICG fluorescence method, ①ICG fluorescence appeared on average 20 seconds after intravenous injection, and ②the ICG fluorescence contrast reached its maximum level on average 30 seconds after appearance of fluorescence.
After that, fluorescence gradually attenuated, and the contrast became unclear. This means that, in a real surgical situation, it is necessary to carry out marking rapidly as soon as ICG fluorescence appears after intravenous injection. However, if the patient’s liver function is normal, it is possible to repeat acquisition of ICG fluorescent images at regular time intervals.
- Keyword
- Content Type